Case Study: Predicting Response to Vedolizumab in Anti-TNF Refractory IBD Patients Using PIMS® Technology.
Breidert, M., Eftekhari, P., Louis, F., Rotoiu, C., Rath, T., Neurath, M. F., & Atreya, R. (2020). Functional Molecular Network Analysis Enables Prediction of Response to Vedolizumab Therapy in Anti-TNF Refractory IBD Patients. Crohn's & colitis 360, 2(2), otaa037. https://doi.org/10.1093/crocol/otaa037
Background
Inflammatory Bowel Disease (IBD), including Crohn's disease and ulcerative colitis, is a chronic condition that significantly impacts patients' quality of life. While biologic therapies like anti-TNF agents have revolutionized treatment, a substantial proportion of patients do not respond to these therapies. Vedolizumab, an anti-integrin antibody, offers an alternative for patients refractory to anti-TNF treatments. However, predicting which patients will respond to vedolizumab remains a challenge, highlighting the need for personalized medicine approaches.
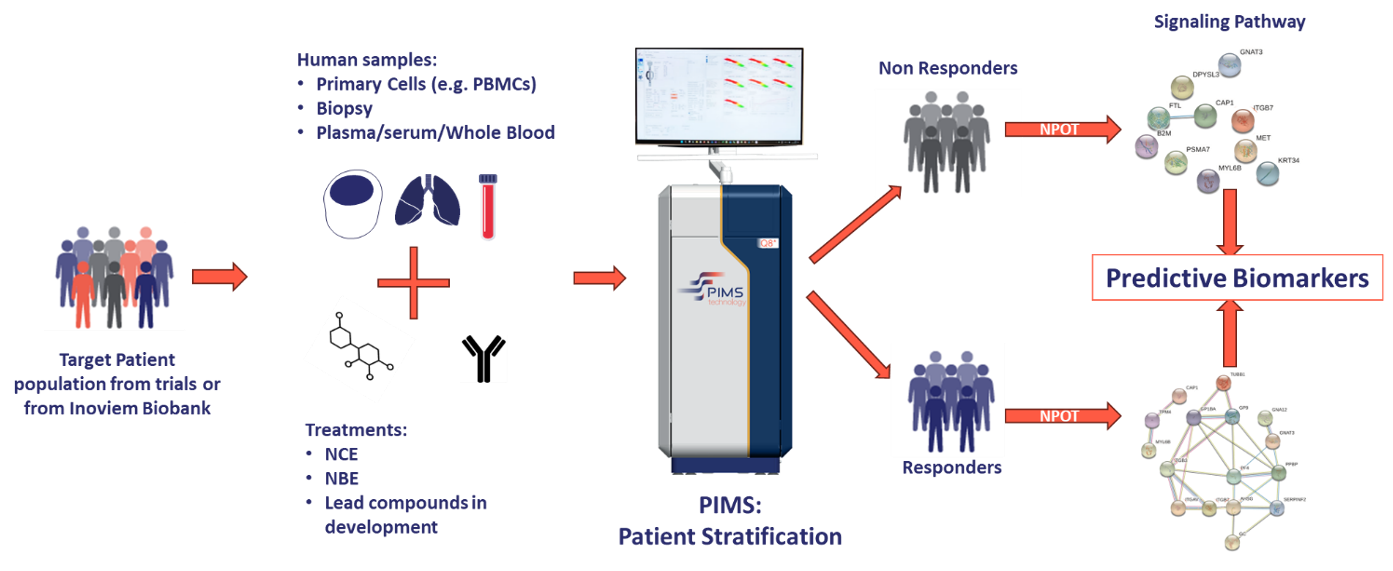
In this case study, we demonstrate how Physiological Intermolecular Modulation Spectroscopy (PIMS®), a patented label-free technology, was used to predict response to vedolizumab in anti-TNF refractory IBD patients with high accuracy. This study, conducted in collaboration with the University of Erlangen-Nürnberg, Germany, and with Takeda Pharmaceuticals Gmbh, showcases the potential of PIMS® in guiding personalized treatment decisions.
Objective
The study aimed to:
- Predict clinical response to vedolizumab in anti-TNF refractory IBD patients using PIMS®.
- Identify underlying molecular networks associated with response using the Nematic Protein Organization Technic (NPOT®).
Methods
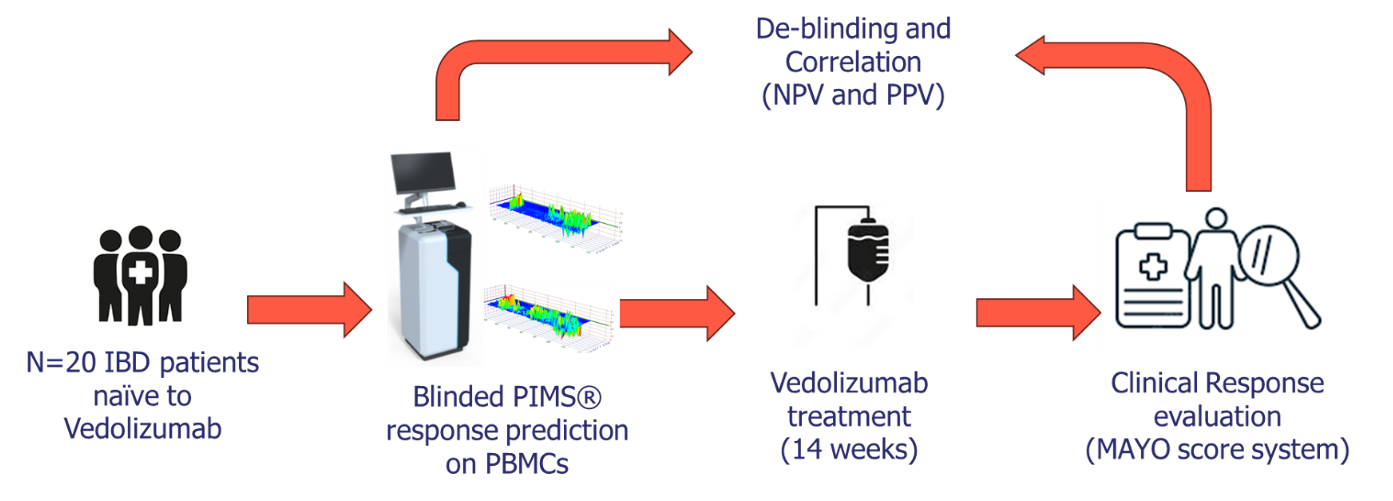
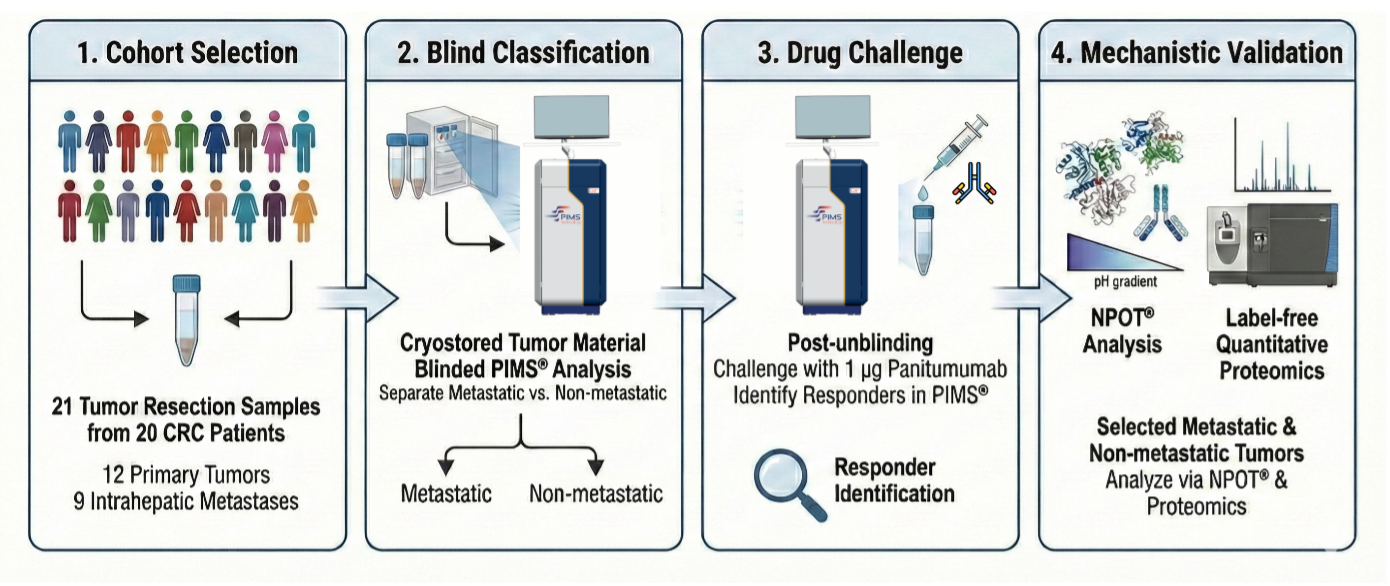
Patient Cohort:
20 BD patients (13 Crohn's disease, 7 ulcerative colitis) who had previously failed at least one anti-TNF therapy were enrolled. Blood samples were collected at baseline (week 0) and week 14 of vedolizumab therapy.
PIMS® Analysis:
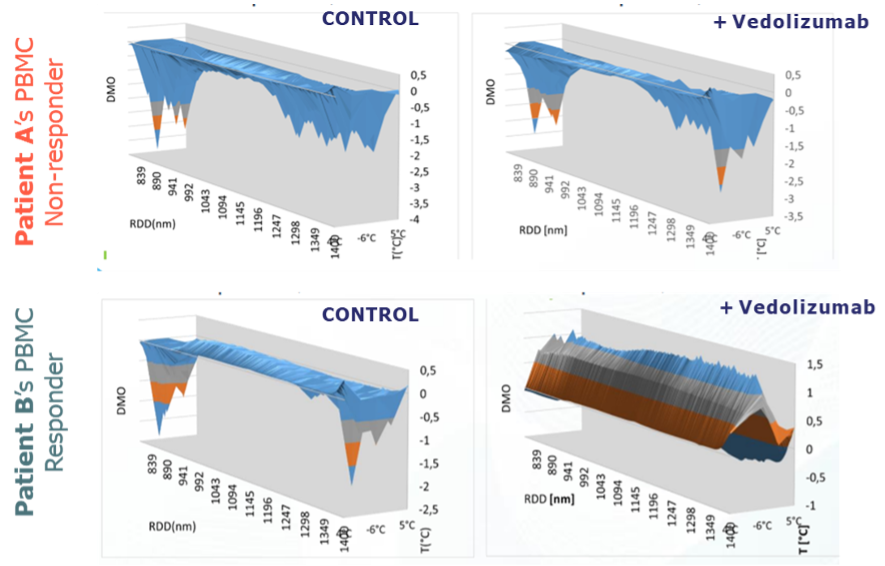
- Peripheral blood mononuclear cells (PBMCs) were isolated and analysed using PIMS®. A spectra using PBMCs without Vedolizumab was obtained for each patient, representing the baseline. Then the same PBMCs were incubated with Vedolizumab and a new spectra have been acquired.
- PIMS® measured changes in water molecule resonance and macromolecular conformation in response to vedolizumab, generating dynamic fingerprints of molecular interactions.
- The Individual Macromolecular Volume (IMV), resulting from the difference between the signal from the test cell and the blank, was calculated to differentiate responders from non-responders.
Clinical Response Assessment:
Response was defined as a reduction in disease activity scores (Harvey-Bradshaw Index for Crohn's disease and partial Mayo Score for ulcerative colitis) at week 14.
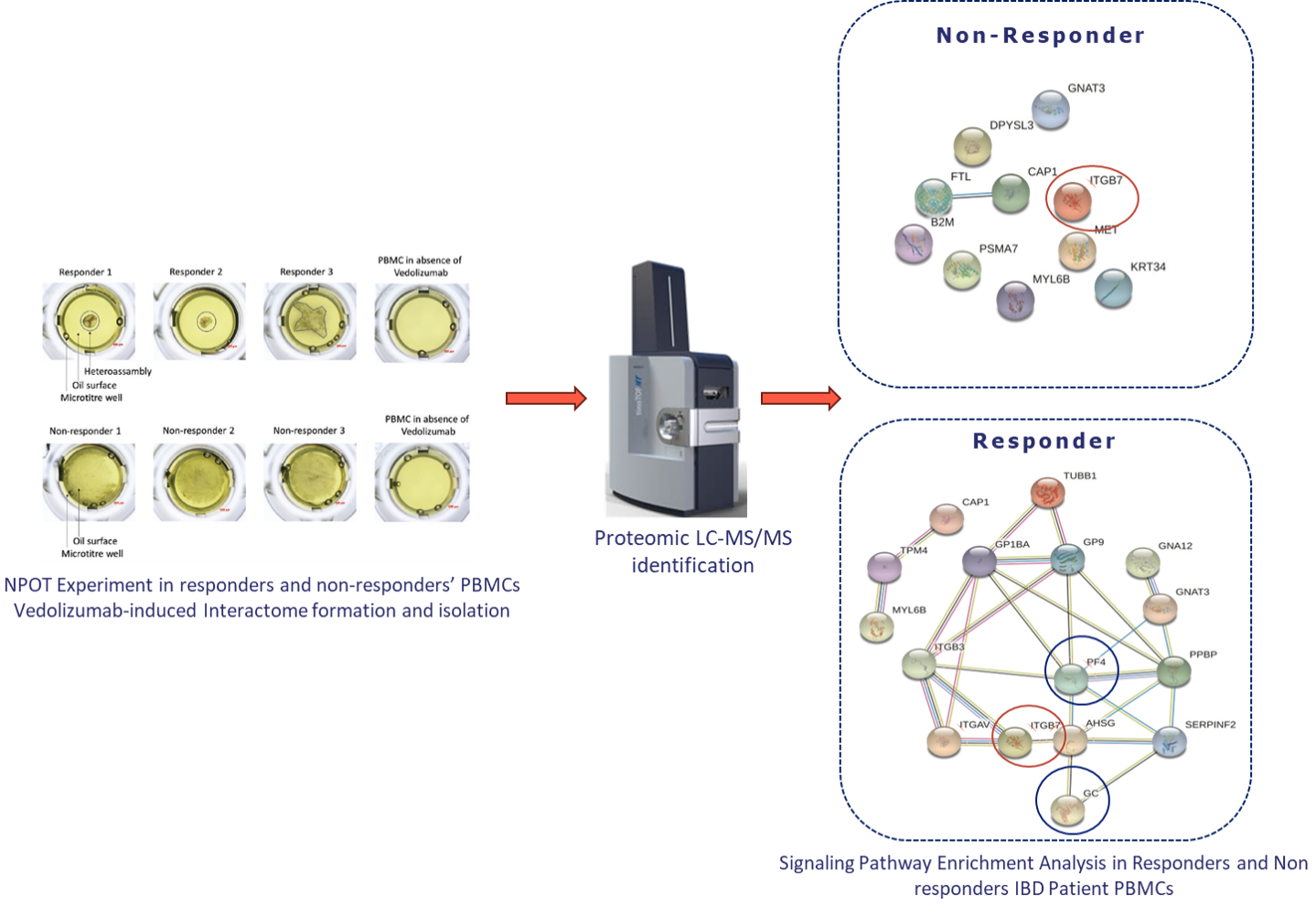
NPOT® Analysis:
Inoviem's NPOT® Platform (www.inoviem.com) was used to identify functional molecular networks in responders by analysing protein-protein interactions induced by Vedolizumab in PBMCs. Proteins were identified using liquid chromatography-tandem mass spectrometry (LC-MS/MS).
Results
PIMS® Predicts Response with High Accuracy:
- PIMS® accurately predicted clinical response to vedolizumab in 100% of ulcerative colitis patients and 77% of Crohn's disease patients.
- Overall, PIMS® achieved an 89% positive predictive value (PPV) and an 82% negative predictive value (NPV) for all IBD patients.
| Patient Group |
Total Patients |
Responders
(Week 14) |
Non-Responders
(Week 14) |
PIMS Accuracy
(Week 0) |
| Crohn's Disease (CD) |
13 |
7 |
6 |
77% |
| Ulcerative Colitis (UC) |
7 |
4 |
3 |
100% |
Overall
(IBD Patients) |
20 |
- |
- |
PPV: 89%
NPV: 82% |
Patient distribution, response rates, and PIMS prediction accuracy for vedolizumab-treated IBD patients.
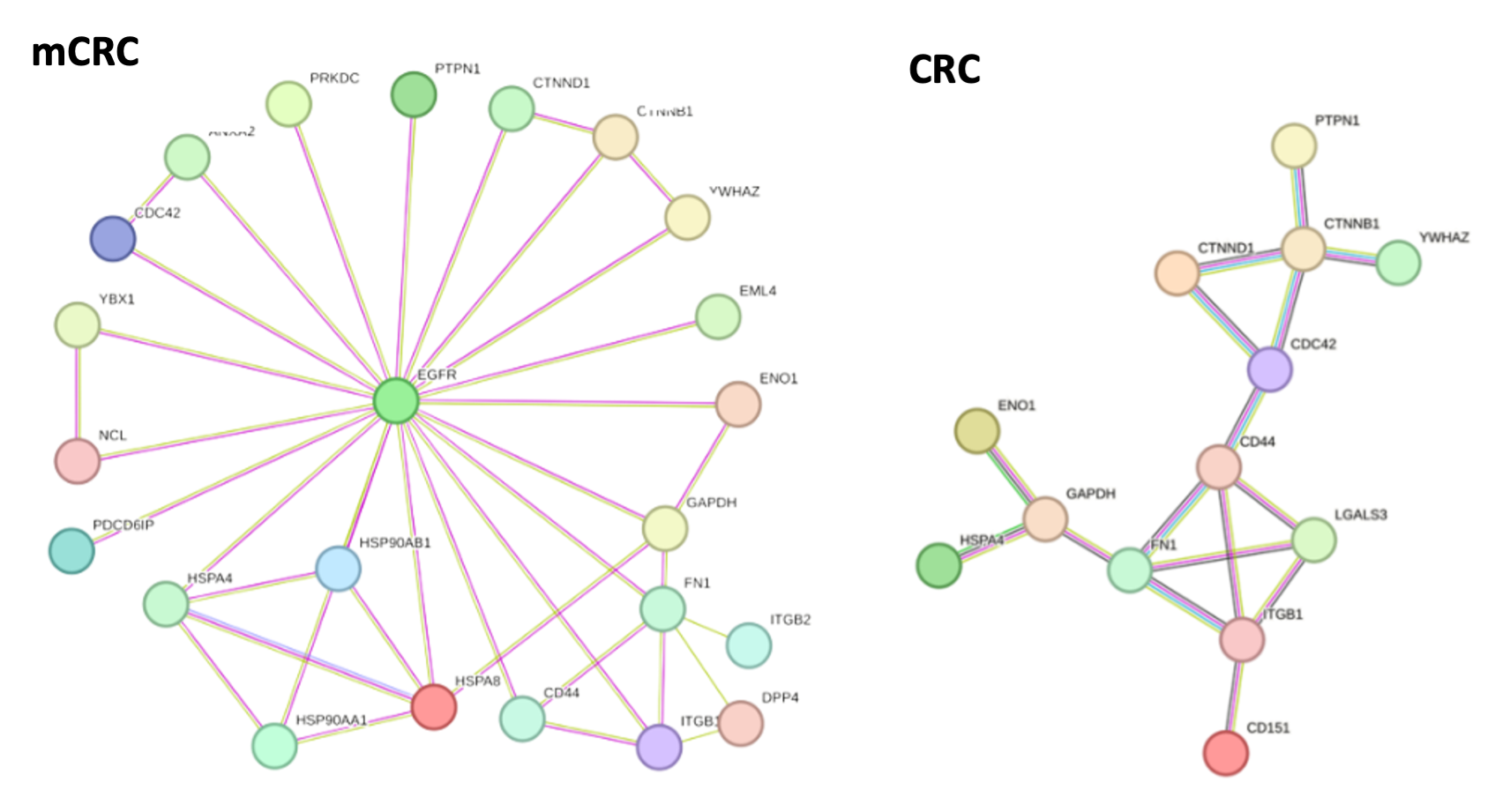
Mechanistic basis for stratification: NPOT® reveals functional molecular networks.
To identify the molecular basis for stratification, we applied NPOT® platform on clinically-confirmed responders and non-responders PBMCs, challenged with Vedolizumab.
In both responders and non-responders, NPOT® identified the primary target of Vedolizumab, α4β7 integrin (ITGB7).
In responders, NPOT® identified a distinct molecular network involving co-receptors of ITGB7, such as ITGAV and ITGB3, which play a role in vedolizumab's mechanism of action. Additionally, NPOT® detected proteins linked to the drug's activity, including PF4 and AHSG.
Non-responders lacked this functional network, explaining their lack of response to therapy.
Key Biomarkers Identified:
Platelet Factor 4 (PF4) and Vitamin D-binding protein (GC) emerged as potential biomarkers for predicting vedolizumab response, offering insights into the molecular basis of treatment efficacy.
Discussion
This study highlights the power of PIMS® in personalized medicine for IBD. By analysing molecular interactions in patient blood samples, PIMS® can stratify patients into responders and non-responders before initiating therapy, enabling more informed treatment decisions. The integration of NPOT® further elucidated the underlying molecular networks, providing mechanistic insights into vedolizumab mode of action.
The identification of PF4 and GC as potential biomarkers opens new avenues for developing companion diagnostics, which could further refine patient selection for vedolizumab therapy.
Low vitamin D levels have been linked to a higher risk of vedolizumab treatment failure in patients with inflammatory bowel disease (IBD). It has been showed that patients with low vitamin D were more likely to experience poor response to the drug, both during the initial treatment phase and over the long term. This suggests that vitamin D levels could serve as a useful marker to predict which patients are more likely to benefit from vedolizumab (Gubatan et al. 2021; Abraham et al. 2023).
Conclusion
PIMS® technology offers a non-invasive, rapid, and highly accurate method for predicting response to vedolizumab in anti-TNF refractory IBD patients. By combining PIMS® with NPOT®, clinicians can not only predict treatment outcomes but also gain insights into the molecular mechanisms driving response, paving the way for truly personalized medicine in IBD.
Why This Matters
- For Patients: PIMS® ensures that patients receive the most effective treatment from the start, reducing unnecessary side effects and improving quality of life.
- For Clinicians: PIMS® provides a reliable tool for treatment decision-making, enhancing the precision of IBD management.
- For Researchers: The integration of PIMS® and NPOT® offers a powerful platform for biomarker discovery and mechanistic studies in drug development.